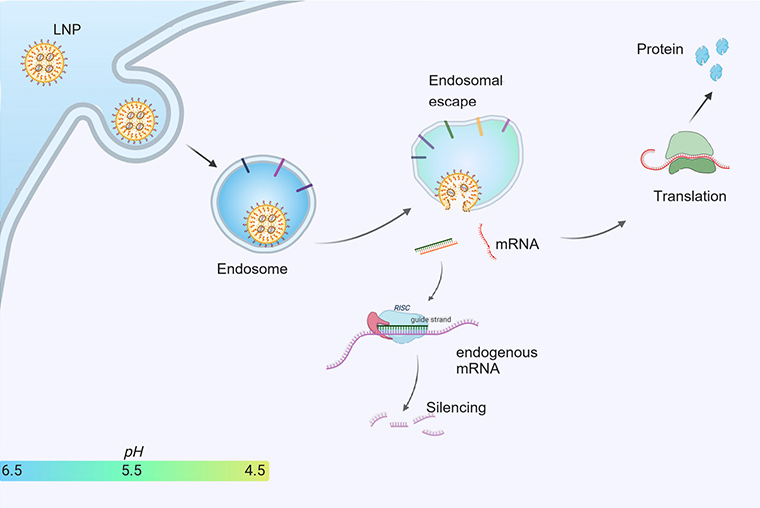
RNA Sequence Design
-
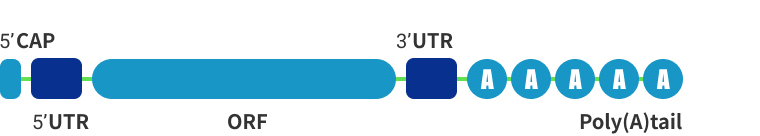
 Optimization of CDS regions using artificial intelligence algorithms
Optimization of CDS regions using artificial intelligence algorithms
-
 Proprietary UTR screening technology for customized UTR design of RNA sequences
Proprietary UTR screening technology for customized UTR design of RNA sequences
-
 Integration of RNA secondary structure prediction tools to enhance RNA structural stability and translation efficiency
Integration of RNA secondary structure prediction tools to enhance RNA structural stability and translation efficiency
i-Core LNP™ Precision Delivery Solutions
i-Core LNP™ Pulmonary Nebulization Delivery System
-
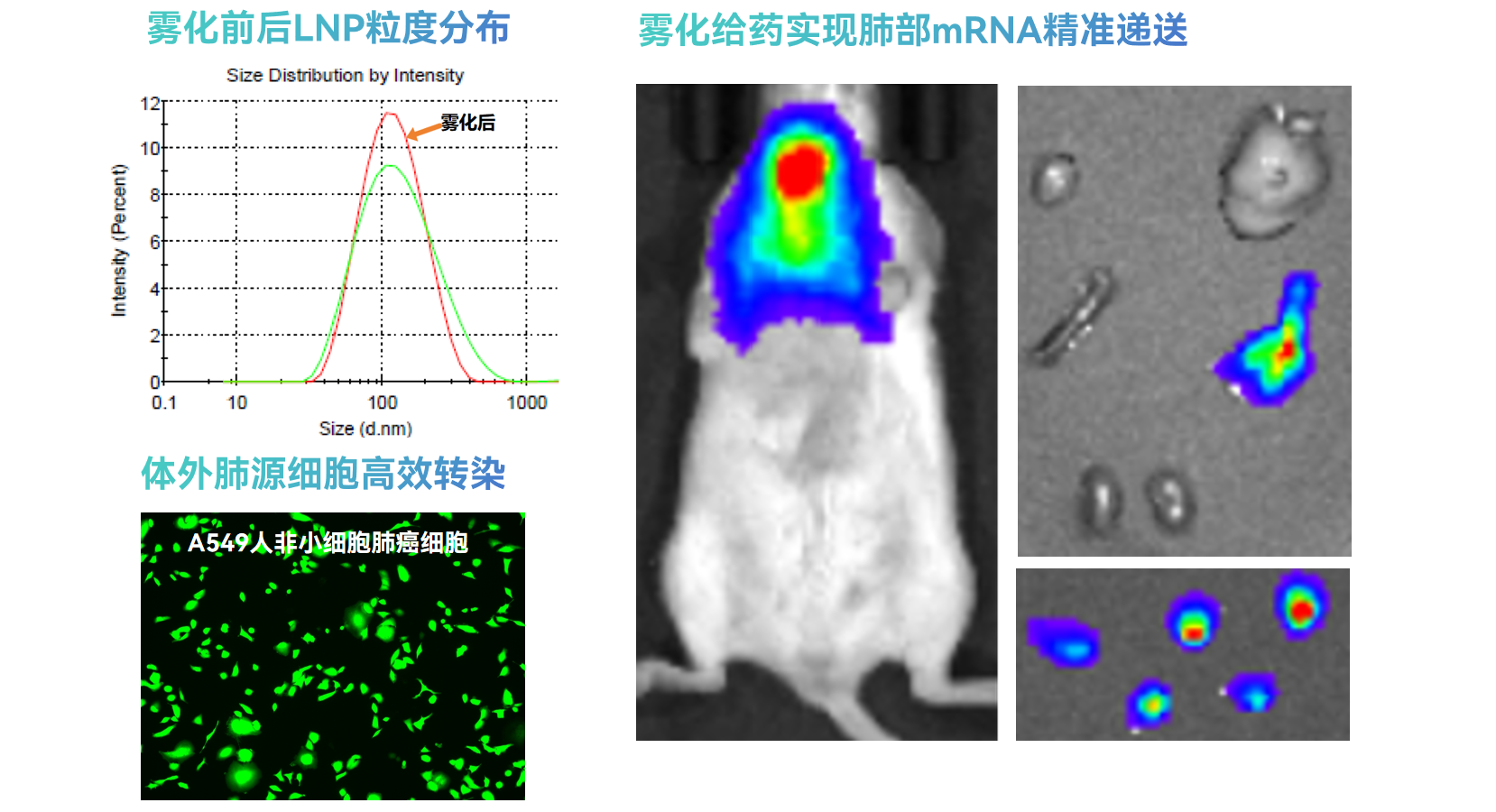
 High Stability Formulation: Unique formulation ensures lipid nanoparticles maintain stability under high-frequency impacts during nebulization. LNP particle characteristics remain consistent before and after nebulization, providing a reliable drug delivery platform.
High Stability Formulation: Unique formulation ensures lipid nanoparticles maintain stability under high-frequency impacts during nebulization. LNP particle characteristics remain consistent before and after nebulization, providing a reliable drug delivery platform. -
 Efficient Pulmonary Delivery: Advanced delivery system precisely and efficiently delivers mRNA/siRNA to target lung cells, ensuring therapeutic molecules reach their sites of action.
Efficient Pulmonary Delivery: Advanced delivery system precisely and efficiently delivers mRNA/siRNA to target lung cells, ensuring therapeutic molecules reach their sites of action. -
 Precise Lung Targeting: Achieves specific organ targeting to the lungs, with loaded RNA expression primarily concentrated in lung tissue, significantly reducing off-target expression in liver/spleen and other non-target organs.
Precise Lung Targeting: Achieves specific organ targeting to the lungs, with loaded RNA expression primarily concentrated in lung tissue, significantly reducing off-target expression in liver/spleen and other non-target organs. -
 Applicability: Through local nebulization administration, this LNP delivery system demonstrates excellent tolerability in mouse/rat animal models, establishing a solid foundation for clinical applications.
Applicability: Through local nebulization administration, this LNP delivery system demonstrates excellent tolerability in mouse/rat animal models, establishing a solid foundation for clinical applications.
i-Core LNP™ Skin Delivery System
-
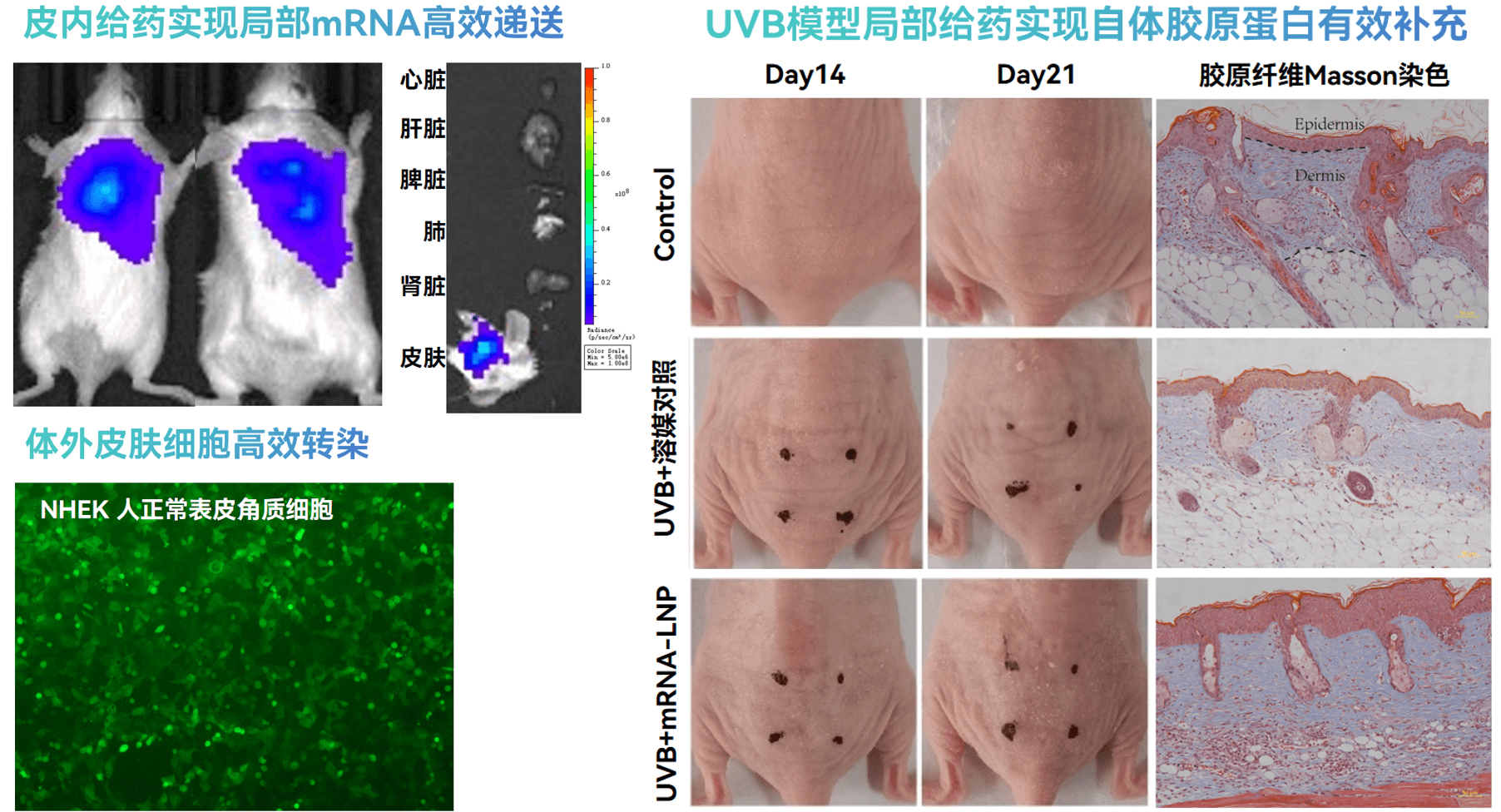
 Superior Skin Targeting Performance: Unique LNP formulation specially designed for local skin treatment. After intradermal administration, mRNA expression is highly concentrated in target skin tissues, effectively avoiding off-target expression in non-target organs such as the liver, achieving precise treatment.
Superior Skin Targeting Performance: Unique LNP formulation specially designed for local skin treatment. After intradermal administration, mRNA expression is highly concentrated in target skin tissues, effectively avoiding off-target expression in non-target organs such as the liver, achieving precise treatment. -
 Significant Anti-Aging Effects: In UVB-induced skin aging models, low-dose COL3A1 mRNA-LNP administered intradermally effectively promotes collagen synthesis and replacement, significantly reducing wrinkle formation, providing an innovative solution for skin anti-aging.
Significant Anti-Aging Effects: In UVB-induced skin aging models, low-dose COL3A1 mRNA-LNP administered intradermally effectively promotes collagen synthesis and replacement, significantly reducing wrinkle formation, providing an innovative solution for skin anti-aging. -
 Excellent Biosafety Profile: Minimal local injection irritation, significantly lower immunogenicity than existing LNP products on the market. Long-term safety studies have not identified spleen or liver toxicity, providing reliable safety assurance for clinical applications.
Excellent Biosafety Profile: Minimal local injection irritation, significantly lower immunogenicity than existing LNP products on the market. Long-term safety studies have not identified spleen or liver toxicity, providing reliable safety assurance for clinical applications. -
 Applicability: Through local administration, this LNP delivery system demonstrates excellent tolerability in mouse/rat animal models, establishing a solid foundation for clinical applications.
Applicability: Through local administration, this LNP delivery system demonstrates excellent tolerability in mouse/rat animal models, establishing a solid foundation for clinical applications.
i-Core LNP™ Vaccine Delivery System
-
 Proprietary IP Formulation System: Self-developed innovative ionizable lipid materials combined with precisely optimized formulation combinations, creating an advanced delivery platform with completely independent intellectual property rights.
Proprietary IP Formulation System: Self-developed innovative ionizable lipid materials combined with precisely optimized formulation combinations, creating an advanced delivery platform with completely independent intellectual property rights. -
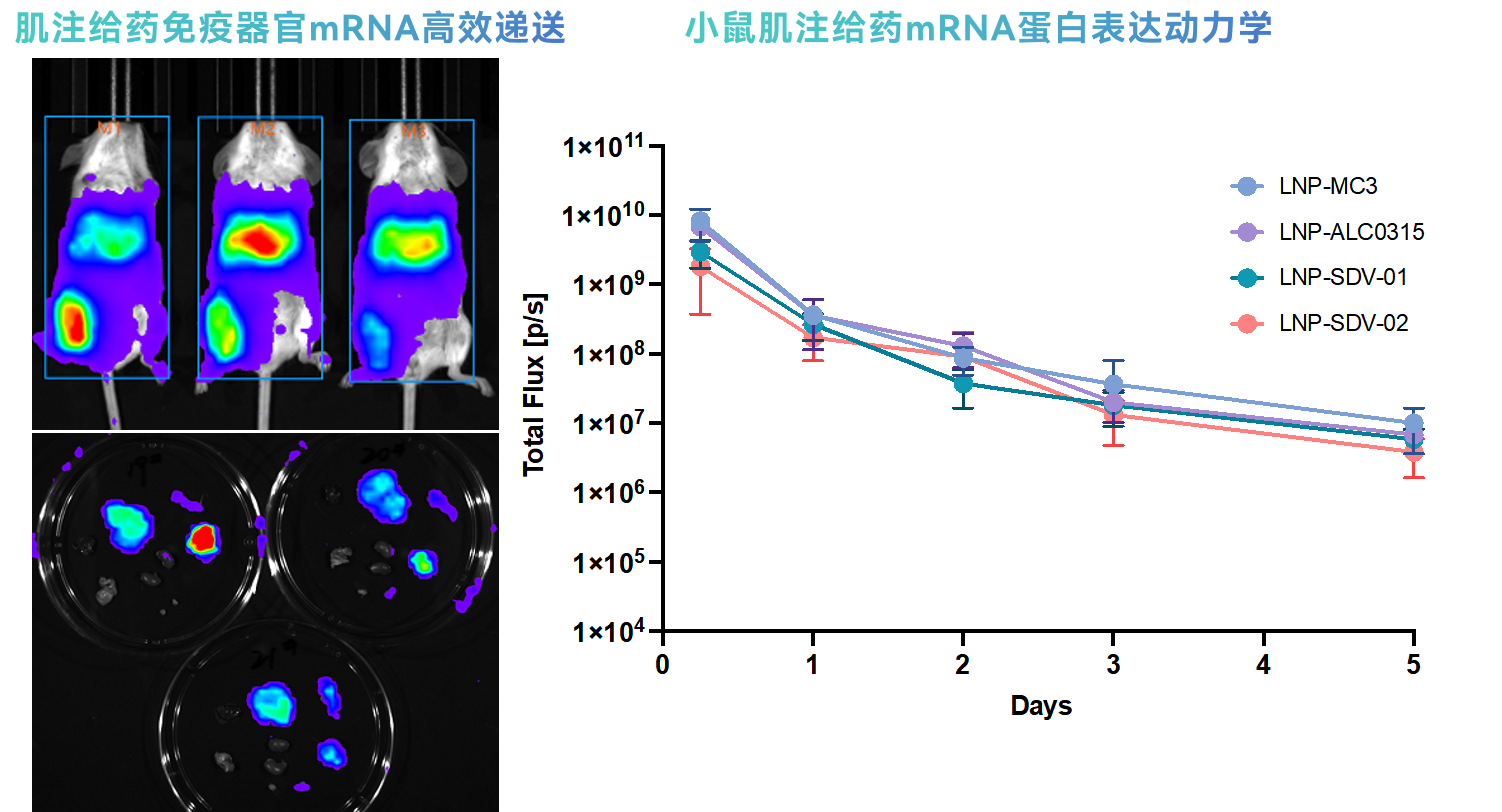
 Efficient Immune Organ Targeting: Following intramuscular (IM) administration, mRNA achieves efficient expression in key immune organs (lymph nodes, spleen). Its tissue distribution characteristics are consistent with internationally marketed products, ensuring maximized vaccine immune response.
Efficient Immune Organ Targeting: Following intramuscular (IM) administration, mRNA achieves efficient expression in key immune organs (lymph nodes, spleen). Its tissue distribution characteristics are consistent with internationally marketed products, ensuring maximized vaccine immune response. -
 Superior Protein Expression Kinetics: The expression kinetics of mRNA-encoded proteins after single administration are fully comparable to internationally marketed products, demonstrating stable and reliable therapeutic effects and lasting immune protection.
Superior Protein Expression Kinetics: The expression kinetics of mRNA-encoded proteins after single administration are fully comparable to internationally marketed products, demonstrating stable and reliable therapeutic effects and lasting immune protection.
SuperLNP™ Pre-formulated Lipid Nanoparticles
-
 SuperLNP™ offers a new model for efficient RNA encapsulation, providing GMP-grade standard pre-formulated LNPs for on-site preparation of RNA-LNP complexes. Compared to traditional methods, SuperLNP™ eliminates the need for complex equipment. It only requires direct mixing with RNA solution before use to achieve efficient encapsulation, greatly shortening RNA-LNP preparation cycles and production costs.
SuperLNP™ offers a new model for efficient RNA encapsulation, providing GMP-grade standard pre-formulated LNPs for on-site preparation of RNA-LNP complexes. Compared to traditional methods, SuperLNP™ eliminates the need for complex equipment. It only requires direct mixing with RNA solution before use to achieve efficient encapsulation, greatly shortening RNA-LNP preparation cycles and production costs.
-
 SuperLNP™ provides a new LNP platform for clinical research and commercial production with higher efficiency and lower costs.
SuperLNP™ provides a new LNP platform for clinical research and commercial production with higher efficiency and lower costs.